Submitting a Research Proposal
Submitting a Research Proposal to FHS
Researchers are encouraged to review the linked guides (below) and detailed information on this page (further below) before submitting to a research proposal to the Framingham Heart Study.
- A Guide for Investigators I – PIs interested in requesting FHS data
- A Guide for Investigators II – PIs interested in collaborating for FHS Gen 3/Omni2 Exam 4
After your research proposal is approved, ancillary study investigators must also review and adhere to our Policies and Procedures.
For questions or comments please contact study administrators at fhs@bu.edu.
How to submit a research application to FHS:
The FHS Research Application is available to all investigators interested in accessing FHS data, materials and approval for research prior to seeking funding.
- An investigator may (1) request an account through the Research Application platform. Upon approval, an email will be sent to the investigator to create their password protect account. Then (2) the investigator will then be able to create an application through the Research Application web-based platform and submit it for review.
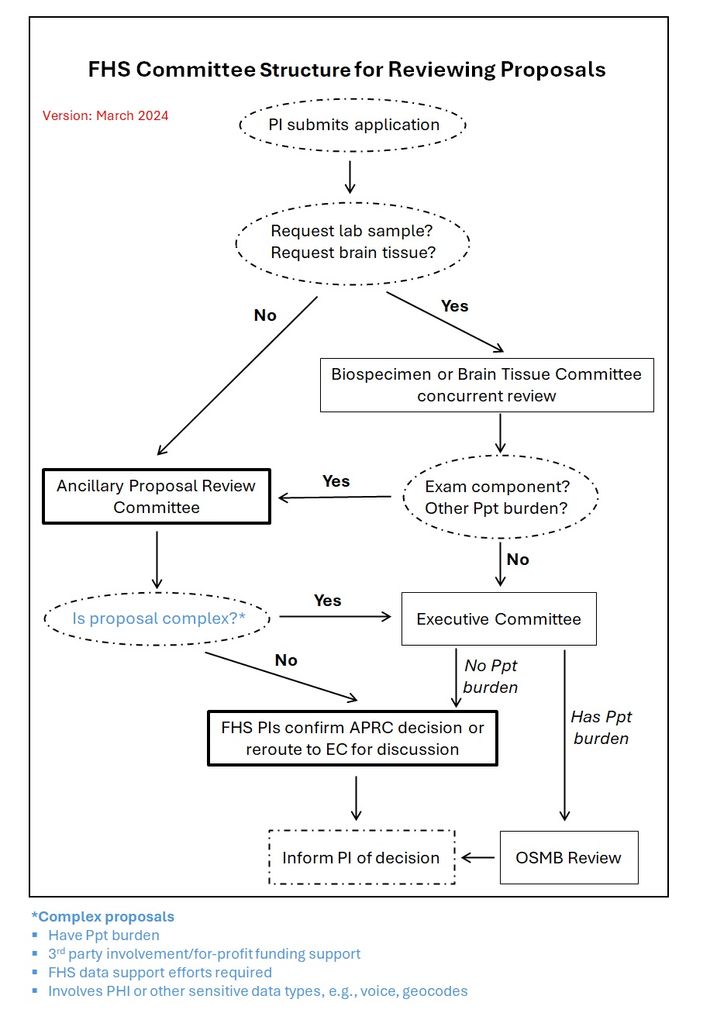
- Following an application’s submission, proposals are routed to one or more research review committees based on the nature of the application.
- See Routing of Applications section below for detail.
- The Biospecimen and External Peer Review Committees provide expert review for applications that will be decided upon by the FHS Executive Committee.
- The FHS Executive Committee provides the final decision.
- All FHS Research Applications (proposals) require “Approval” before investigators may submit to sponsors for funding.
- General Review Committees’ Considerations
- Does the proposal require the unique characteristics of the FHS cohort(s)?
- Is collaboration with a Framingham investigator planned?
- What Cohort(s) and Exam cycle(s) are required?
- What Framingham data and/or analyses are needed?
- Does the proposal show proof of resources for conducting the project?
- Does the proposal put minimal demand on FHS resources?
- Does the proposal follow FHS recommendation to include in the design the Omni cohorts that are parallel to Offspring and Third Generation cohorts? Exclusion of the corresponding Omni cohort requires a specific scientific justification.
Applications that plan to seek funding
The Executive Committee reviews applications for project that plan to seek sponsorship (e.g. outside funding). The ancillary study must provide the funds needed for successful completion of the study. The need for such support must be stressed in research grant applications.
For FHS executive committee review: Submit application by the 3rd Monday of the month for review the same month. Submit at least 6-8 weeks before your potential sponsors’ grant deadline to insure time for correspondence, clarifications and modifications.
Ancillary Study Costs may include, but are not limited to:
- Staffing for data collection and analyses
- Subject recruitment if outside of main exams
- FHS expense incurred by performing data manipulations
- On site rental of research exam, lab, and office space if required
- Personnel, equipment and supplies for the project
- Cost of notification of reportable results
Request for Participant Contact
The FHS Executive Committee reviews requests for participant contact.
Executive Committee Considerations
- What is the expected burden to participants? Time and discomfort expectations.
- When will data be collected?
- Is follow-up needed? Specify length of time and events to be followed up on.
- Consent must be obtained from each subject in the ancillary study.
- Does the ancillary study interfere with or hamper participation in the main study?
- A copy of the signed ancillary study consent form for every individual participant is to be included in the FHS record. A file tracking all signed ancillary consent forms is to be maintained by the ancillary study and an electronic copy of that file is to be delivered to FHS.
- The FHS strongly urges that proposals include the use of Omni specimens and data in any study whenever the necessary covariates are available. Because of the smaller size of the Omni, the Executive committee will not require that power calculations be provided for the Omni.
Requests for Clinical Data
The Research Committee reviews requests for clinical data, including exam chart review and imaging data. A Framingham liaison and biostatistician needs to be named on the application, and of particular importance is whether the proposal will require assistance from Framingham’s Data Management team and the extent of the burden. FHS Service Center Fees may apply.
Requests for Biological Specimens for Genetic Research
The Biospecimen Committee reviews applications for DNA or other biological specimens for use in genetic studies. DNA is available from the Framingham Study participants for whom lymphoblast cell lines have been established. All DNA samples will be distributed in 96 well plates at a concentration of 10ng/ul unless other specifications are provided. Currently, there are five standard plate sets: Gen1 plate set is the Original Cohort, Gen 2A and Gen 2B plate sets are the Offspring Cohort, the NOS plate set contains spouses of Offspring, and the Gen 3 plate set is the Third Generation Cohort. Omni Cohort plate sets made from blood DNA are also available. Requests for DNA will be filled from these plate sets. A small set of samples without cell line back up are available with additional approval from the Executive Committee.
Custom DNA plate sets may be requested. For example, a sample set of persons with CHD and age and sex matched controls may be requested. Custom DNA requests require detailed justification. Custom DNA requests are filled when the lab has time available and there is no guaranteed timeline.
Fee Structure for Data and Materials
Biospecimen Committee Review Considerations
- A clear description of the aims and of the significance of the study
- The proposed study design
- The analysis plan and power calculations that justify sample size
- The justification for the use of Framingham specimens
- A description of the gene/polymorphism/mutation to be studied
- The experience of the recipient laboratory in this or similar assays
- The justification for the amount of DNA requested
- The FHS strongly urges that proposals include the use of Omni DNA specimens in any study whenever the necessary covariates are available. Because of the smaller size of the Omni, the Biospecimen Committee will not require that power calculations be provided for the Omni.
Addendum DNA Application
The Addendum Application allows investigators to extend the scientific aims of a previously approved application. An addendum application may request to add elements to a previously approved application or to follow up on the original application’s resultant data through additional analyses, assays, or subjects. Approved addendum applications require a new distribution agreement to cover the new elements of the proposal. An addendum is not the appropriate tool for resubmission of applications that require edits either prior to or after committee review. Those applications should simply be edited and resubmitted, retaining the same ID number. Addendum applications to the Biospecimen Committee must be submitted within 3 years of the effective date of the original proposal’s Distribution Agreement or within 3 years of the approval letter date, whichever comes later.
Expedited DNA Application
The Expedited DNA Application is available for rapid turn-around to follow up on genetic results, such as genome-wide association study (GWAS) results and may be submitted at any time. Permission will only be granted if the preliminary data for follow-up has reached genome-wide significance. The phenotype in the initial GWAS must be clearly described, with a description of the analogous trait in the Framingham Heart Study. Careful justification of the relevance of the requested phenotype is required. Requests for non-renewable specimens may not use the expedited pathway. Use of extant genetic material may be requested through this mechanism. Expedited DNA Applications will be reviewed and a Decision Letter sent to investigators within 10 business days. Applications for other types of follow-up on genetic results may be considered for expedited review on a case by case basis. The investigator needs to provide adequate justification of the need for expedited review.
Requests for Biological Specimens for Non-genetic Research
The FHS Biospecimen Committee evaluates proposals requesting biological specimens for non-genetic research (requests for biological specimens for genetic studies are also reviewed by the Biospecimen Committee). Specimens are available from the Original Cohort, Offspring Cohort, NOS (New Offspring Spouses), Gen 3 Cohort and the two Omni Cohorts.
The Omni cohorts consist of men and women reporting African-American, Hispanic, Asian, Indian, Pacific Islander and Native American origins. Omni Cohort 2 included many individuals related to the participants of Omni Cohort 1 and also individuals unrelated to Omni Cohort 1 members. These samples were collected following the same laboratory protocols used for the concurrent Offspring and Third Generation examinations. Exam components also follow the same protocols, starting with the first exams of each Omni Cohort. The FHS strongly urges that proposals include the use of Omni specimens in any study whenever the necessary covariates are available. Exclusion of the Omni Cohort requires a specific scientific rationale.
Applications will be judged on their scientific merit and the availability of samples. Please direct questions regarding biological specimen availability to Dr. Joel Henderson, Laboratory Director, at 508-935-3477. Applications are reviewed by the FHS Executive Committee after approval by the Biospecimen Committee.
Biospecimen Committee Considerations
- Credentials and qualifications of laboratory performing analysis
- Suitability of analysis for a community-based cohort
- Demonstrated reproducibility of measurement
- Relevance to themes of FHS research, with a priority for NHLBI-related research
- If clinically actionable measurement, a plan to refer lab values back to FHS
- Availability of the biologic material that is requested
Distribution of biological specimens requires:
- A signed Data and Materials Distribution Agreement (DMDA) and IRB Approval
- Agreement to perform the approved assay directly from the tubes shipped from FHS
- Agreement to promptly provide all assay results to FHS such that:
- The investigator provide all results for all tests and duplicate results
- The investigator may not delete results considered to be unusual or “outliers”
- The investigator return results in the Excel spreadsheet that was initially provided with participant ID numbers and test date
- Agreement to return any residual specimens without delay
- Agreement to pay for the preparation of samples should it constitute an unusual burden
- Agreement to return resultant assay data to Patrice Sutherland, FHS Lab Manager.
- Agreement to communicate individual “notifiable” results to the Executive Committee. Investigators will be informed if a proposed assay may yield reportable results.
- Investigators further need to be aware that sample recipients are randomly requested to participate in a quality control program designed by the FHS.
Modifying an existing, approved application
To modify an application that has already been approved by the Executive Committee, investigators are requested to fill out a Modification Request Form and submit it to FHS@bu.edu